cameroon gce june 2000 chemistry Paper 2
TO DOWNLOAD CAMEROON GCE june 2000 chemistry Paper 2 CLICK ON THE LINK BELOW
JUNE 2000
SECTION A
-
The table gives some information about six elements, U, V, W, X, Y and Z. These letters are NOT the usual symbols of the elements. Using only the letters U-Z answer the questions that follow.
Element |
AtomicNumber |
ElectronicConfiguration |
U |
2,8/18,7 |
|
V |
11 |
1 |
w |
2,8,14,2 |
|
X |
2,8,4 |
|
Y |
18 |
|
z |
17 |
-
Give :
(i) The atomic number of U………
(ii) The electronic configuration of Z (2marks)
(b) Which one of the elements in the table does not form compounds?
Give a reason for your answer………………………………….
-
Which two of the elements in the table belong to the same group of periodic table? Give the name of the group.
Elements ………group. ……………………. (2marks)
-
d) (i) Write tire formula of the compound formed between X and Z ………..
(ii) Write the formula of the sulphate of W… (2marks)
-
From the elements U-Z select the element which
Is a transition metal………………………………………….. (2marks)
-
What type of compound is formed between V and
Hydrogen?……………………………………………… (1 mark)
2.
Sodium hydroxide is manufactured industrially by electrolysis.
(a) Name the electrolyte used………………….. (1 mark)
-
State the material of which each electrode is made
Up. Cathode………….. …anode ……………. (2marks)
-
Write an ionic equation for the reaction by which
Sodium hydroxide is produced…………………. (2marks)
-
Name two by-products of the process and give one large scale use of each by-product
-
How would sodium hydroxide pellets be obtained
From tire solution…………………….. ……………… (1 mark)
-
On heating 5g of granulated zinc with excess 2MHC1 (aq) V cm3 of hydrogen gas was produced in 5 minutes
-
Why is the HCL (aq) used in excess? …………. (1 mark)
(b)The table below shows the other conditions under which the reactions take place. Complete the table by stating whether V increases, decreases or remind the same. In each case give the reason.
Experiment condition |
Results |
Reasons |
i. 5g of powder zinc and excess 2M HO (aq) heated for 5 minutesii. 5g of granulated zinc an d excess 2MHQ (aq) heated for 5tnanuiesiii. 5g of granulated zinc and excess 2M HG (aq) at room temperature |
(c)(i) Write the equation for the reaction…………………
(ii) if the reaction continued until all the zinc is used up, calculate the volume of hydrogen produced measured at room temperature and atmospheric pressure (3marks)
4.
 B
B
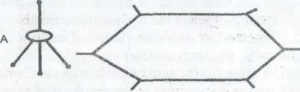
Structure A and B above represent two crystalline forms of carbon
-
Name the crystalline form represented by
-
A ……………………………………. (1 mark)
-
B:……………………………………………………… (1 mark)
-
What term is used to describe this phenomenon
Exhibited by carbon?……………………………….. (1 mark)
(c) Complete the table below to bring out the differences in
The physical properties given
Property |
A |
B |
Reason forDifference. |
Hardness |
|||
Electrical conduction |
(d) How would you show by experiment that A and B are different forms of the same elements?
………………………………………………… (2marks)
(e) Give one use of each of the two crystalline forms. A…………B……… (2marks)
-
Given below are the molecular formula of some <
Hydrocarbons A: C4H10B:C2H4 C: C2H2 D: CH4 E: C6H6
-
Name the hydrocarbons A,B,C and E
AM: .B C…… E…………………………….. , (2marks)
-
From A-I| select the hydrocarbon that is
-
Obtained by decarboxylation of sodium ethanoate (Sodium acetate)
-
The main component of kitchen gas in Cameroon
-
Used as a monomer in the production of an
Important plastic……………… L……….
-
An aromatic hydrocarbon………………… “(2marks)
-
State two conditions required for the formation
Of a hydrocarbon plastic ….. (2marks)
-
What is the structural difference between A and
B?………………………………………………….. ………. (2marks)
-
How would B be obtained from a named alcohol?
6.
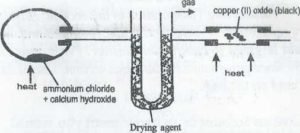
A mixture of ammonia um chloride and calcium hydroxide was heated in a round bottom flask as shown on the diagram above. The gas produced was dried and passed over heated copper (ii) oxide in a tube.
-
a) Write the equation for the reaction in the round bottom flasks ……… (2marks)
-
Identify
(i) The gas………………………….
(ii) The drying agent………………………………… (2marks)
-
State any color change that would be observed
In the heated tube………………………………. …… (1 mark)
-
Give the products formed when the gas is passed over heated copper (II) oxide….. .(3marks)
-
Give two large scale uses of the gas produced (2marks)
SECTION B
Answer any two question in this section. All questions carry equal marks. Where appropriate, equations and diagrams should be given to clarify your answer. Write your answers on the lined s which follow this section. Useful data will be found on the back.
7.
Give an account of an experiment you would
carry out to determine the heat of combustion of methanol (your description should end with the collection of data) Carrying out such an experiment it was found that temperature of the 200cm3 of water was raised from 20°C to 75°C when 2.10g of methanol was completely burnt. Use the data above to determine the heat of combustion of methanol (25marks)
-
Briefly describe how aluminum and iron can be
Extracted from their ores. For each metal give two large scale uses (25marks)
-
Describe the type of bonding that exists in each of the following substances:
Magnesium chloride, Carbon dioxide and Calcium. Give two physical properties of each substance and relate each property to the type of bonding found in the substance
10.
Cu(g) +4HN03(aq —————————-> Cu(N03)2(aq) +2N02(g) + 2H20
NaOH(aq) + HCl(aq) ———–à NaCL(aq) + H20(l)
BaCL2(aq) + K2SO4 ————————à BaSO4(s) + 2KCL(aq)
The three equations given above represent three different
Methods of preparing salts in the laboratory. In each case describe
how a pure solid sample of the salt in bold print indicated in the
Equation can be obtained from the given starting substances
(25marks)