A SIMPLE VIEW OF ATOMIC STRUCTURE
Hello everyone today we are going to discuss ideals about atomic structure that you may have seen back in your chemistry class rooms .
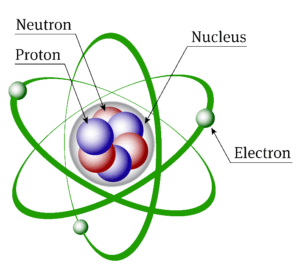
The sub-atomic particles
We have 3 sub-atomic particles Protons, neutrons and electrons.
| relative mass |
charge | |
| proton | 1 | +1 |
| neutron | 1 | 0 |
| electron | 1/1836 | -1 |
Remark : Beyond A-level: Protons and neutrons don’t in fact have exactly the same mass – neither of them has a mass of exactly 1 on the carbon-12 scale (the scale on which the relative masses of atoms are measured). On the carbon-12 scale, a proton has a mass of 1.0073, and a neutron a mass of 1.0087
The behavior of protons, neutrons and electrons in electric fields:
One way to distinguish between these particles is their behavior in the electric field .
Protons are positively charged and so would be deflected on a curving path towards the negative plate.
Electrons are negatively charged and so would be deflected on a curving path towards the positive plate.
Neutrons don’t have a charge, and so would continue on in a straight line.

The nucleus
The nucleus is at the center of the atom and contains the protons and neutrons. Protons and neutrons are collectively known as nucleons.
Virtually all the mass of the atom is concentrated in the nucleus, because the electrons weigh so little.
TAKE Note from here !!
Working out the numbers of protons and neutrons
Number of protons = ATOMIC NUMBER of the atom
The atomic number is also given the more descriptive name of proton number.
Number of protons + number of neutrons = Mass number of an atom
The mass number is also called the nucleon number.
This information can be given simply in the form:

For the example above can you give me the proton number, neutrons number , atomic number and mass number ?
Solution
For the proton number is easy we just count = 2 ( which is the same as atomic number )
For the neutron number will do some little math, we know that mass number = protons + neutrons , right ?
Then from there we can say that mass number – protons = neutrons
Therefore neutrons = 4 -2 = 2,
For the mass number you can just read it above 🙂 .
NB: is to note that the neutron number is not always the same as the proton number is just a coincidences that it happened to be the same .
The atomic number is tied to the position of the element in the Periodic Table and therefore the number of protons defines what sort of element you are talking about. So if an atom has 8 protons (atomic number = 8), it must be oxygen. If an atom has 12 protons (atomic number = 12), it must be magnesium.
Similarly, every chlorine atom (atomic number = 17) has 17 protons; every uranium atom (atomic number = 92) has 92 protons.
Isotopes And Isotopy.
It can happened that the number of neutron can vary within small limits , and this is called isotopy . Isotopy is a phenomenon whereby atoms of an element have the same atomic number but different mass number. The difference in mass number is due to the different number of neutrons. Since we are talking about isotopy we can’t leave out isotopes which the simplest form can be define as “Isotopes are the elements that have the same atomic number (number of protons) and different mass number (number of neutrons)”.
NB: we shall take the calculation part later
Examples of isotopes
| protons | neutrons | mass number | |
| carbon-12 | 6 | 6 | 12 |
| carbon-13 | 6 | 7 | 13 |
| carbon-14 | 6 | 8 | 14 |
The electrons
Working out the number of electrons
Atoms are electrically neutral, and the positiveness of the protons is balanced by the negativeness of the electrons. It follows that in a neutral atom:
Number of electrons = number of protons
So, if an oxygen atom (atomic number = 8) has 8 protons, it must also have 8 electrons; if a chlorine atom (atomic number = 17) has 17 protons, it must also have 17 electrons.
The arrangement of the electrons
The electrons are found at considerable distances from the nucleus in a series of levels called energy levels. Each energy level can only hold a certain number of electrons. The first level (nearest the nucleus) will only hold 2 electrons, the second holds 8, and the third also seems to be full when it has 8 electrons and so on .
To work out the electronic arrangement of an atom
- Look up the atomic number in the Periodic Table – making sure that you choose the right number if two numbers are given. The atomic number will always be the smaller one.
- This tells you the number of protons, and hence the number of electrons.
- Arrange the electrons in levels, always filling up an inner level before you go to an outer one.
e.g. to find the electronic arrangement in chlorine
- The Periodic Table gives you the atomic number of 17.
- Therefore there are 17 protons and 17 electrons.
- The arrangement of the electrons will be 2, 8, 7 (i.e. 2 in the first level, 8 in the second, and 7 in the third).
So to summaries what we saw today,
We see that the different part of the atom are really important in O and A level chemistry , please everyone should try to understand these before going ahead .
If you liked the notes please say thanks below to encourage us 🙂
Special Thanks to chemguide















nephropathy
October 5, 2017
Clinical studies prove new remedy to be effective lowering GFR in KIDNEY DISEASE patients http://renalimpairedfunction.blogspot.com/2015/03/how-to-improve-kidney-function.html
ekobe godwin otteh
November 27, 2017
all you notes are ammezing .simply and understanding. thank you people a lot.
Tar
November 6, 2023
Thanks to chemguide