Starting material for synthesis
Many of the organic chemicals synthesized in industry rely on cruel oil, vegetable oils or fats for their starting materials. The cracking of alkanes in crude oil produces large quantities of short-chain alkanes have anything from two to eight carbon atoms. They include ethene (CH2 = CH2) and propene (CH3CH=CH2) and can be converted into alcohols, ketones, acids and other derivatives by appropriate reactions.
The hydrolysis of naturally occurring fats and oils provides an important source of carboxylic acids. These carboxylic acids contain between eight and eighteen carbon atoms but they are restricted to compounds with an even number of carbon atoms.
Figure 2 below shows some important synthetic routes based on the availability of alkenes from petrochemicals and the availability of carboxylic acids from naturally occurring fats and oils. This overall scheme contains many of the reactions included in the summaries at the end of the organic chemistry chapters.
Look closely at figure 2. Notice that alkenes, alcohols and carboxylic acids are the starting points for several reactions. These compounds are often the starting point of many organic syntheses. The cracking of crude oil fractions a ready supply of alkenes for industry while fats and oils can be used to manufacture alcohols and carboxylic acids. Primary alcohols and carboxylic acids can be interconverted via aldehydes using suitable oxidants and reductants
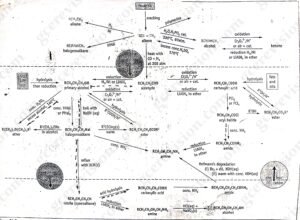
Figure 2: Important synthetic roues in organic chemistry















Valérie Tsala
January 7, 2025
No correction for technical section
Valérie Tsala
January 7, 2025
No correction for technical sections