cameroon gce june 2004 chemistry Paper 2
To DOWNLOAD CAMEROON GCE JUNE 2004 CHEMISTRY Paper 2 click on the link below

JUNE 2004
SECTION A
1. Ethene, an unsaturated hydrocarbon produced by the complete dehydration of ethanol under suitable conditions,
a) Define
i) unsaturated
ii) hydrocarbon.
iii) Dehydration 3 marks
b) Give the reaction conditions necessary for the
complete dehydration of ethanol to ethEne… 3marks
a) Write the equation for the complete dehydration of ethanol to ethene .3 marks
b) Complete the table below:
|
Ethanol
|
Ethene
|
Empirical Formula
|
|
|
Structural Formula
|
|
|
c) What products would be obtained when ethene is reacted with hydrogen in the presence of a nickel catalyst? 1 mark
2. The diagram below c an be used for the separation of mixtures
a) Usually a few pieces of broken porcelain are
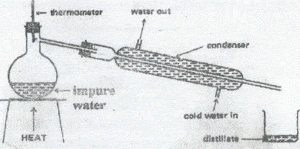
placed in the flask containing impure water. What purpose does the porcelain serve? (1 mark)
Why is the thermometer placed in the position shown? 1 mark
b) Why is it wrong to reserve the water connections
of the condenser? …. l mark
c) The distillate is called distilled water
(i) Identify two gaseous impurities that it might
contain . 1 mark
(ii) Why is it impossible for the distillate to contain
salt?. 3marks
d) Suggest TWO reasons why the apparatus would be unsuitable for separating crude oil into its different components 2marks
e) Name TWO other methods of separating liquid/liquid mixtures 2marks
3. The table below concerns elements A, B and C. A, B and C are their usual symbols.
|
Elements
|
Element
|
Element
|
|
A
|
B
|
C
|
Atomic Number
|
11
|
6-
|
|
Number of protons
|
|
|
16
|
Number of neutrons
|
12
|
6
|
|
Mass Number
|
|
12
|
32
|
Electronic
|
2,8,1
|
|
2,8,6
|
configuration
|
|
|
|
(a) Complete the table.
(b) Which of the elements A, B and C is a compound (lmark)
(c) Element A combines with element C to form a compound.
(i) Write down the formula of the compound
(ii) What type of bonding exist in the compound?
(iii) Draw a dot and cross diagram of the compound
(3marks)
4. In the diagram, an electric current was passed through aqueous solutions of copper (II) sulphate, silver nitrate and lead (II) nitrate connected in series. Inert electrodes were used and the current in the circuit was measured

What does A and B represent in the diagram
A:
B:
2marks
(b) The same gas was produced at one of the electrodes i n each solution. Identify
(i) the electrode
(ii) the gas
(c) A metal coating was deposited on the other
(d) electrode in each solution. Write an ionic equation to show what happens in each solution 2marks
during this time (3marks)
(d)If 2 amperes are passed through the circuit for 4 minutes. Calculate.
(i) the number of coulombs that have passed
(ii) the number of grams of lead that will be deposited
(5) Water can be described as hard or soft.
(a) What is “hard water”? (l mark)
(b) After a long time inside of a kettle or a boiler appears white
(i) What is the cause of this whitness?
(ii) How can this whitness be removed? (2marks)
(c) Complete the table below by giving the two types of hardness in water and their causes.
(d) State one
(i) advantage of hard water and disadvantage of hard water (2marks)
(ii) Give one source of hard water (l mark)
6. The following elements lithium (Li), Sodium (Na), Potassium (K) belong to the Periodic Table.
(a) State one
(i) physical property common to this group of
elements
(ii) chemical property common to this group of
elements 2 marks
(b) These elements react even with cold water.
(i) Give two observations when a small piece of sodium is added to cold water..
(ii)In what way does lithium differ from sodium in
its reaction with cold water? (4marks)
(c) Like the others lithium forms a carbonate.
(i) Write down the formula of lithium carbonate.
(ii) Suggest whai would happen if lithium carbonate
were strongly heated (3marks)
SECTION B
Answer TWO questions. One objective of this section is to give you the opportunity to organize material and present ideas, including calculations and diagrams where appropriate, in a clear and logical form.
7. Describe an experiment you would carry out to determine the heat of precipitation of silver chloride. Your description should end with the collection of data.. In one such experiment 50cm3 of molar silver nitrate reacted with 50cm3 of molar potassium chloride solution and a temperature rise of 4.0 °C was observed.
Use these data to determine the molar heat of precipitation of silver chloride.
State why the calculated value is different from that found in standard textbooks. (25marks)
8. Write short notes on the following types of chemical reactions giving examples
(a) Addition reactions
(b) Substitution reactions
(c) Esterification
(d) Cracking
9. Three of the factors that affect the rate at which a reaction proceeds include temperature, the surface area of the reactants and catalyst. For each of these factors describe an experiment to illustrate its effect on the rate of reaction. (25marks)
10. Describe simple tests for the positive identification of the chemicals:
(a) Ammonium sulphate
(b) Potassium sulphate
(c) Iron (II) chloride (25m arks)












