ATOMIC ORBITALS AND Electronic Configurations
Hello once more today we are going to talk about atomic orbitals , excited ? then lets get started ! Before you start you need to know the basic of an electron that’s the sub atomic particles , But Thank God I have some notes on it , you can read them Here
What is an atomic orbital?
Orbitals and orbits
When a planet moves around the sun, you can plot a definite path for it which is called an orbit. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact inhabit regions of space known as orbitals.
Orbits and orbitals sound similar, but they have quite different meanings. It is essential that you understand the difference between them.
The impossibility of drawing orbits for electrons
To plot a path for something you need to know exactly where the object is and be able to work out exactly where it’s going to be an instant later. You can’t do this for electrons.
The Heisenberg Uncertainty Principle says – loosely – that you can’t know with certainty both where an electron is and where it’s going next. (What it actually says is that it is impossible to define with absolute precision, at the same time, both the position and the momentum of an electron.)
That makes it impossible to plot an orbit for an electron around a nucleus. Is this a big problem? No. If something is impossible, you have to accept it and find a way around it.
Important Note about orbitals
Before I move further I will like to point out some little things out for better understanding over the years people have always painted a picture of an atom as a plant with electron orbiting it , but to simply say that picture is wrong !
To make it a bit clear and simple to understand electrons are moving around nucleus not in a circular manner ! A bit confusing right ? 🙂 just follow up you will understand as we move down promise .

Note: In this diagram (and the orbital diagrams that follow), the nucleus is shown very much larger than it really is. This is just for clarity.
Suppose you had a single hydrogen atom and at a particular instant plotted the position of the one electron. Soon afterwards, you do the same thing, and find that it is in a new position. You have no idea how it got from the first place to the second.
You keep on doing this over and over again, and gradually build up a sort of 3D map of the places that the electron is likely to be found.( which explains that there is not a clear pattern for the electron there for explaining what I was trying to say above)
In the hydrogen case, the electron can be found anywhere within a spherical space surrounding the nucleus. The diagram shows a cross-section through this spherical space.
95% of the time (or any other percentage you choose), the electron will be found within a fairly easily defined region of space quite close to the nucleus. Such a region of space is called an orbital.
Electronic Configurations
As we saw before an atomic orbital shows where there is a 95% chance of finding a particular electron.Basically there are four types of atomic orbitals , s,p,d, and f which all have have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons.
Rules for Assigning Electron Orbitals
Occupation of Orbitals
Electrons fill orbitals in a way to minimize the energy of the atom. Therefore, the electrons in an atom fill the principal energy levels in order of increasing energy (the electrons are getting farther from the nucleus). The order of levels filled looks like this:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p
One way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce this pattern. Another way is to make a table like the one below and use vertical lines to determine which subshells correspond with each other.
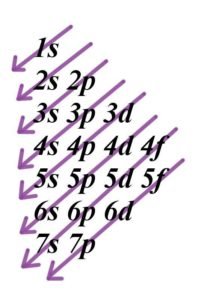
“Electrons-in-boxes”
Orbitals can be represented as boxes with the electrons in them shown as arrows. Often an up-arrow and a down-arrow are used to show that the electrons are in some way different.
A 1s orbital holding 2 electrons would be drawn as shown on the right below
![]()
but it can be written even more quickly as 1s2. This is read as “one s two” – not as “one s squared». You mustn’t confuse the two numbers in this notation:
![]()
Examples on electronic configuration
Example 1 : Nitrogen
If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, a very important element in the biology of plants using our S notation
we get 1s2 2s2 2p3 .
Using the box we get .
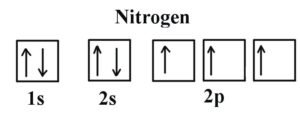
We can clearly see that p orbitals are half-filled as there are three electrons and three p orbitals. This is because Hund’s Rule states that the three electrons in the 2p subshell will fill all the empty orbitals first before filling orbitals with electrons in them. If we look at the element after Nitrogen in the same period.
Okay ?? 🙁 no I guess let get another example .
Example 2 Oxygen :
Oxygen (Z = 8) its electron configuration is: 1s2 2s2 2p4 (for an atom)
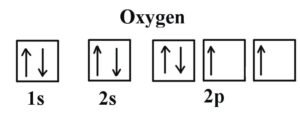
Oxygen has one more electron than Nitrogen and as the orbitals are all half filled the electron must pair up.
As assignment try the following and give me your answers in the comments
- vanadium (V, Z=23)
- Aluminum (Z=13)
- Cobalt (Z=27)
If you liked the notes please say thanks below to encourage us
Special Thanks to chemguide, chem.libretexts.org/














