cameroon gce june 2006 chemistry Paper 2
To DOWNLOAD CAMEROON GCE JUNE 2006 CHEMISTRY Paper 2 click on the link below

JUNE 2006
SECTION A
1. Chlorine and Bromine are members of Group VII of the Periodic Table of elements.
(a) What is the name of this group of elements?
(lmark)
(b) Give any other member of this group and give if s physical state at room temperature and atmospheric pressure.
Name……: physical state.
(c) The atomic number of chlorine is 17.
(i) What is its electronic configuration?
(ii) By means of a diagram, show the electron arrangement in a chlorine molecule 2marks
(d) A jet of ammonia is spontaneously flammable in chlorine, producing nitrogen and hydrogen chloride.
(i) Write an equation for this reaction..
(ii) What will be observed if excess ammonia is used?
Explain your answer (4marks)
(iii) Give ONE industry use of chlorine (I mark)
2. This question concerns the behavior of ions in different media.
(a) Describe what happens if a few drops of sodium hydroxide are added to:
(i) Copper (II) sulphate solution
(ii) Aluminium sulphate solution, ( 4marks)
(b) What happens if excess sodium hydroxide solution is added to: ,
(i) a(i)above? –
Explain:…..
a(ii) above?
Explain: (4marks)
(c)Give the products formed when dilute sulphuric acid reacts with magnesium carbonate……. (2marks)
3. Hydrocarbons are either saturated or unsaturated.
(a) What do you understand by the term
unsaturated hydrocarbon? (2marks)
(b) Name one unsaturated hydrocarbon and draw its structural formula (2marks)
(c) Give the name and formula of the product
formed when bromine and the unsaturated hydrocarbon combine (2marks.)
(d) (i) Name one saturated hydrocarbon and draw its formula
(ii) What would be the products of the reaction between 1 mole of this hydrocarbon in d (i) above
and 1 mole of bromine? (2marks)
(a) (i) Which of the two named hydrocarbons above
can be converted to a polymer? ..
(ii) Name the polymer and draw its structure ( 2marks)
4.
Carbon monoxide can be prepared as shown below
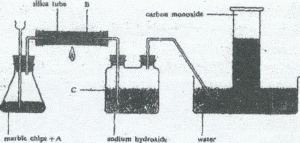
(a) (i) Give a suitable reagent represented by A
(ii) What is solid B
(iii) What is the function of sodium hydroxide
solution? ,..(3marks)
(b) Write an equation for the reaction taking place in:
(i) the flask
(ii) the silica tube
(iii) the two neck bottle . (4marks
(c) Apart from the laboratory and industry, carbon monoxide is produced from other sources.
(i) Name one such source
Explain how it is formed ………………. ( 2 marks)
(d) Why is carbon monoxide poisonous? ……(I mark)
5.
5.900cm3 of a sample of dry air was passed over heated copper turnings in a Hard glass tube and the ensuing gas was collected.
(a) What component of the air sample was absent in
the collected gas? . (I mark)
(b) Assuming the complete removal of the
component in (a),What volume of gas was collected at the end of the experiment? (2marks)
(c) In an experiment 36g of an oxide of copper was reduced to the metal by passing hydrogen gas over the heated oxide, 32g of copper remained
(i) Calculate the possible formula for the copper
oxide..
(ii) Write an equation for the reaction taking place between the oxide of copper and hydrogen gas
(iii) What mass of water was formed? (2marks)
6. An element X is in Group I of Periodic Table of elements. X reacts with chlorine to form a white solid.
(a) What type of bonding is likely to be present in
the white solid? (I mark)
(b) If a concentrated solution of this white solid was electrolysed, what would be the main products discharged at the electrodes?
(i)Write equations for the reactions that take place (4marks)
(c) Write the formula for:
(i) the nitrate of X ?.
(ii)the carbonate of X ………………………. (2 marks)
(d) What would be the effect of heat on:
(i) the nitrate of X
(ii) the carbonate of X
(iii) write an equation for the change (3marks)
SECTION B
7. a) Giving the raw materials and stating the chemical
principlesinvolved, briefly describe the industrial preparation of sulphuric acid and sodium hydroxide. (b)GIVE ONE laboratory use and THREE industrial uses of suphuric acid and of sodium hydroxide . (25mks)
8. Chemical substances are held together by three types of chemical bonds which are known as ‘covalent, ionic’ and metallic. Choose a substance in each case whose atoms are held by one of these types of bonds and describe how the bonding occurs.
Give ONE property of each substance and relate this to the type of bonding in the substances (25marks)
9. What is a standard solution? Describe how you
would prepare a 0.1 M solution of sodium carbonate and use it to determine the molarity of a solution of hydrochloric acid. (25 marks)
10.The rate of production of hydrogen by the reaction of magnesium with 0.5M hydrochloric acid was investigated and the following results were obtained at a temperature of 15°C and 1 atmosphere pressure using 0.0669g of magnesium ribbon. The hydrogen gas was collected over water in a trough.
Time in minutes
|
0
|
0.5
|
1.0
|
1.5
|
2.0
|
2.5
|
3.0
|
3.5
|
4.0
|
4.5
|
5.5
|
Total volume of H2 in cm3 ( at 15 c at 1 atm)
|
9.5
|
18.0
|
56.5
|
33.5
|
41.4
|
48.0
|
54.0
|
59.0
|
62.0
|
63.0
|
63.0
|
(a) (i) Plot a graph of volume of hydrogen (vertical axis) against time (horrizontal axis).
(ii) On the same graph , sketch and label the curve you would have expected if the hydrochloric acid had been heated to 25° C before the experiment.
(iii) What is the total volume obtained from 0.0669g of magnesium at 15°C according to this experiment?
(iv) Calculate the volume that would have been obtained at this temperature from 1 mole of magnesium.
(v) How does your answer compare with the 24000cm3 normally accepted for the molar volume of gas? Give one possible reason for the discrepancy you have found.
(b) When a similar experiment was conducted with pieces of calcium cabomate instead of magnesium, a value of 16.800 cm3 for the molar volume of carbon dioxide was found.
(i) Why do you think the value was so low ?
(ii) State and explain two ways in which the rate of reaction of calcium carbonate with hydrochloric acid could be increased, other than by heating
(25marks)















Jhon
January 4, 2018
thanks
Jhon
January 4, 2018
<3
Dac
December 20, 2021
Thanks tmfor all this informations