Ascending and descending a homologous series
Ascending and descending a homologous series
The other key reagents in preparing organic aliphatic chemicals are halogenoalkanes and nitriles. Indeed, the conversion of halogenoalkane to nitrile provide an important means of increasing the number of carbon atoms in molecules. So, nitriles allow us to ascend a homologous series. For example, ethanol can be converted to propan-1-ol via propanitrile along the synthetic route shown in figure 3 below.
Another important process of ascending a homologous series is the ‘oxo’ reaction Alkanes, on heating under pressure with carbon monoxide and hydrogen, give aldehydes with one more carbon atom.
RCH=CH2 + CO + H2 RCH2CH2CHO
The aldehyde can then be readily reduced to a primary alcohol. Consequently, this route provides an important process for the manufacture of primary alcohols.
The last few paragraphs have focused our attention on means of ascending a homologous series. How can we reserve this process and descend a homologous series? The crucial reaction in this case is called Hofmann’s degradation. In this reaction an amide is first treated with bromine and dilute potassium hydroxide and then warmed with concentrated alkali. The amide is converted to an amine with one less carbon atom.
RCONH2 + Br2 + 4KOH RNH2 + 2KBr + K2CO3 + 2H20
Overall, the process involves removing the C = O group from the amide to give the amine
RCONH2 —-> RNH2
Figure 3.1 below shows a sequence of reaction which could be used to descend a homologous series from ethanol to methanol


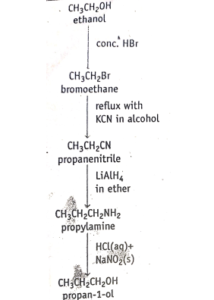
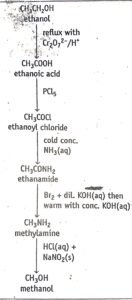
fig 3.1 descending a homologous series







