Hello once more today we are going to me talking about electronegativity . what electronegativity is, and how and why it varies around the Periodic Table. It looks at the way that electronegativity differences affect bond type and explains what is meant by polar bonds.
What is electronegativity
Definition
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons
Fluorine is the most electronegative element is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.
To study electronegativity in details we shall be taking a case study of two atoms A and B ( in which A and B don’t represent any element in particular in the periodic table)
What happens if two atoms of equal Electronegativity bond together?
Consider now our two atoms, A and B. Each atom may be forming other bonds as well as the one shown but these are irrelevant to the argument.
![]()
If the atoms are equally electronegative, both have the same tendency to attract the bonding pair of electrons, and so it will be found on average half way between the two atoms. To get a bond like this, A and B would usually have to be the same atom. You will find this sort of bond in, for example, H2 or Cl2 molecules.
What happens if B is slightly more electronegative than A?
B will attract the electron pair rather more than A does.
![]()
That means that the B end of the bond has more than its fair share of electron density and so becomes slightly negative. At the same time, the A end (rather short of electrons) becomes slightly positive. In the diagram, “” (read as “delta”) means “slightly” – so + means “slightly positive”, and this kind of covalent bond is know as polar bonds
Defining polar bonds
A polar bond is a covalent bond in which there is a separation of charge between one end and the other – in other words in which one end is slightly positive and the other slightly negative. Examples include most covalent bonds. The hydrogen-chlorine bond in HCl or the hydrogen-oxygen bonds in water are typical
This sort of bond could be thought of as being a “pure” covalent bond – where the electrons are shared evenly between the two atoms.
What happens if B is a lot more electronegative than A?
In this case, the electron pair is dragged right over to B’s end of the bond. To all intents and purposes, A has lost control of its electron, and B has complete control over both electrons. Ions have been formed.
![]()
A “spectrum” of bonds
The implication of all this is that there is no clear-cut division between covalent and ionic bonds. In a pure covalent bond, the electrons are held on average exactly half way between the atoms. In a polar bond, the electrons have been dragged slightly towards one end.
How far does this dragging have to go before the bond counts as ionic? There is no real answer to that. You normally think of sodium chloride as being a typically ionic solid, but even here the sodium hasn’t completely lost control of its electron. Because of the properties of sodium chloride, however, we tend to count it as if it were purely ionic.
Lithium iodide, on the other hand, would be described as being “ionic with some covalent character”. In this case, the pair of electrons hasn’t moved entirely over to the iodine end of the bond. Lithium iodide, for example, dissolves in organic solvents like ethanol – not something which ionic substances normally do.
Patterns of electronegativity in the Periodic Table
The most electronegative element is fluorine. If you remember that fact, everything becomes easy, because electronegativity must always increase towards fluorine in the Periodic Table.
Trends in electronegativity across a period
As you go across a period the electronegativity increases. The chart shows electronegativities from sodium to chlorine – you have to ignore argon. It doesn’t have an electronegativity, because it doesn’t form bonds.
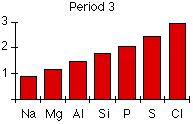
Trends in electronegativity down a group
As you go down a group, electronegativity decreases. (If it increases up to fluorine, it must decrease as you go down.) The chart shows the patterns of electronegativity in Groups 1 and 7.
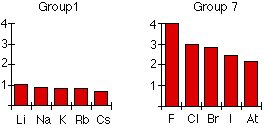
Factors affecting Electronativity :
Electronativiy highly depends on the following factors :
-the number of protons in the nucleus;
-the distance from the nucleus;
-the amount of screening by inner electrons.
– screening or shielding
Why does Electronegativity increase across a period?
Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Think of sodium chloride as if it were covalently bonded.
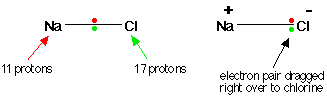
Both sodium and chlorine have their bonding electrons in the 3-level. The electron pair is screened from both nuclei by the 1s, 2s and 2p electrons, but the chlorine nucleus has 6 more protons in it. It is no wonder the electron pair gets dragged so far towards the chlorine that ions are formed.
Electronegativity increases across a period because the number of charges on the nucleus increases. That attracts the bonding pair of electrons more strongly.
Why does electronegativity fall as you go down a group?
Think of hydrogen fluoride and hydrogen chloride.

The bonding pair is shielded from the fluorine’s nucleus only by the 1s2 electrons. In the chlorine case it is shielded by all the 1s22s22p6 electrons.
In each case there is a net pull from the center of the fluorine or chlorine of +7. But fluorine has the bonding pair in the 2-level rather than the 3-level as it is in chlorine. If it is closer to the nucleus, the attraction is greater.
As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus.
Okay now let’s test your understanding
Question time
- Define electronegativity.
- a) On the Pauling scale the electronegativities of nitrogen and oxygen are respectively 3.0 and 3.5.
Why is oxygen more electronegative than nitrogen?
- b) On the same scale, the electronegativity of sulphur is 2.5. Why is sulphur less electronegative than oxygen.
3.By thinking about where the following atoms are in the Periodic Table, sort them into order of increasing electronegativity:
aluminium
barium
boron
caesium
calcium
carbon
fluorine
4. Cl2 CsCl MgCl2 NaCl PCl3 SCl
a) Which of these substances has non-polar bonds? Explain your reasoning.
b) Which of these substances is the most ionic? Explain your reasoning.
c) Suggest a substance which has polar covalent bonds. Explain your reasoning
5. Using the compounds CHCl3 and CCl4 as examples, explain the difference between a polar bond and a polar compound
Special thanks to www.chemguid.uk.org







