cameroon gce june 2001 chemistry Paper 2

TO DOWNLOAD cameroon gce june 2001 chemistry Paper 2 click on the link Below
JUNE 2001
SECTION A
1. Hydrogen can be prepared in the laboratory by reacting hydrochloric acid with Zinc metal.
How do you test for the gas? (2marks)
(a) State any two factors that can make the reaction
go faster. ,. (2marks)
(b) In a reaction between zinc and excess.
1. Hydrogen can be prepared in the laboratory by reacting hydrochloric acid with Zinc metal.
hydrochloric acid at room temperature, 4800 cm3 of hydrogen was collected. Calculate the mass of zinc needed to produce this volume of hydrogen
.. (3marks)
(i) Give the method of collection of hydrogen……..
(ii) On what property of hydrogen does its collection
depend? (2marks)
(e) Give one important use of hydrogen gas (1 mark)
2. Alkenes belong to a homologous series of unsaturated hydrocarbons.
(a) Give the names and structural formulae of the first two members of the series.
(b)Briefly describe a confirmatory test for the members of this series (2marks)
(C)The first member of this series can be converted to a synthetic polymer
(i) Name the polymer
(ii) Give one condition that is needed for the production of the polymer ( 2marks)
(d) Give two uses of the polymer (2marks)
3. The elements X, Y, and Z belong to the same group of the periodic table. Study the table below which shows the reaction of each element dissolved in Carbon tetrachloride with aqueous Potassium chloride, potassium bromide and potassium iodide solutions.
Element I CCL4
|
reaction with Kl(aq)
|
Reaction with
KBr (aq)
|
Reaction with KCU
|
X
|
No change
|
No change
|
No change
|
Y
|
Colourless solution turns dark
|
Colourless solution turns
red
|
No change
|
Z
|
Colourless solution returns dark brown
|
No change
|
No change.
|
(a) Which one of the elements is
(i) the most reactive?
(ii) The least reactive? (2marks)
(b) from the observations, write an equation for
(i) Y and Br
(ii) Y and KL
(iii) Z and Kl(aq) (3marks)
(c) Give the name of the group of the periodic table to
Which these elements belong
(d) Identify the elements represented by: X,Y,and Z . ( 3 marks)
(e) What is the physical state of Z at room temperature? (1 marks)
4.
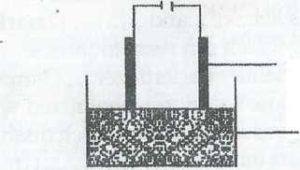
FRIST LINE : Copper electrodes
SECOND LINE : Copper (II) sulphate solution
In the experimental set up above, a current of 0.5A was passed through copper (II)sulphate solution between copper electrodes for 1 hour 30 minutes.
(a) Write equations for the reactions taking place at
the electrodes: At cathode
At anode (2marks)
b) Calculate the quantity of electricity in Faradays use (2marks)
c) Calculate the mass of copper that will be
deposited (2marks)
d) If graphite elects odes have been used instead, identify the product that would be obtained at:
(i) Cathode:
(ii) anode (2 marks)
e) Still using graphite electrodes instead of copper,
what two observations would be made? (2marks)
5. From the list of compounds:
Hydrogen chloride iron (III) chloride
Cone, sulpuric acid lead nitrate
Magnesium oxide Ammonium chloride
Ammonia gas washing soda (Na2 CO3 10H2O)
Select:
(a) a compound that should be added into water but not water into it. (1 mark)
(a) A compound which reacts with an acid to give a
salt and water only (1 mark)
(b) A compound that is efflorescent (1 mark)
-(d) Two compounds that can react to form a
compound from this list. …….and (2marks)
(e) Two compounds which can react to form a
fertilizer and …Identify the fertilizer (3marks)
(f) A compound whose solution when mixed with
hydrogen chloride gives a precipitate which dissolves on heating and appears on cooling… (l mark)
(g) A compound whose solution gives a reddish
brown gelatinous precipitate with aqueous – sodium hydroxide (l mark)
6. Below are five elements arranged in alphabetical order?
Copper (Cu), iron (Fe), magnesium (Mg), silver (Ag), and sodium (Na).
(a) Using just the chemical symbols of the elements,
arrange them in the order in which they are found in the electrochemical series (2 marks)
(b) Which one of these elements is always found free
in nature? (l mark)
(c) A piece of Magnesium ribbon is placed in copper (H) nitrate solution,
(i)What is observed? (2marks)
Explain observation
(ii) Write an equation for the change observed (4marks)
(d)Iron reacts with both steam and dilute
hydrochloric acid. Write an equation for the reaction
(i) iron and steam..
(ii)Iron and hydrochloric acid (2marks)
(e) Which one of these elements reacts vigorously with
cold water? Write an equation for the reaction (2marks)
SECTION B
Answer any TWO questions in this section. All questions carry equal marks. Where appropriate, equations and diagrams should be given to clarify your answer. Write your answer to the lined s which follow this section.
Useful data will be found on the back cover
7. (a) Describe the process for the manufacture of soap and detergents.
(b) State the advantages detergents have over soap (25marks)
8. Describe an experiment to show how the heat of
Combustion of ethanol can be determined, indicating all precautions to be taken (25marks)
9. Using suitable examples in each case distinguish dearly between the following terms as used in chemistry.
(a) Isotropy and allotropy
(b) Condensation polymerization and addition polymerization
(c) Thermal decomposition and thermal dissociation
(d) Neutralization and esterification.
(e) Dehydration and hydrogenation………..(25marks)
10. Describe the type of bonding found in
(a) potassium chloride
(b) carbon tetrachloride
(c) Iron
(d) phosphorus Oxychloride ( 25 marks)